Tissue Engineering and Regenerative Medicine
Fabrication and Optimization of 3D Structures and Methods for Tissue Engineering Applications
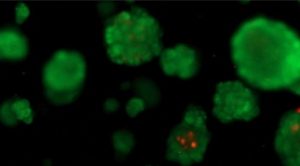
Tissue engineering is an important field of research for tumour and disease modelling, drug screening, and regenerative medicine studies. Due to limitations of conventional two-dimensional (2D) cell culture in mimicking native tissues, recently three-dimensional (3D) cell culture models have started to be utilized.
Several state of the art technologies are being utilized for this purpose in our laboratory. Studies on both scaffold based and scaffold free 3D cell culture applications are run simultaneously; to investigate the potentials of said applications.
Our research group employs biomimetic approaches to overcome current difficulties in tissue engineering field. Motivations of this work ranges from basic fundamental questions to bio-based applications; such as understanding biological functions and responses, developing high-throughput drug screening platforms, or producing next generation regenerative medicine technologies.
Development of Biomaterials for Tissue Engineering Applications
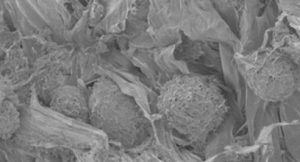
In recent years, novel biomaterials have emerged as promising scaffold materials in tissue engineering field. Materials that can closely mimic extra cellular matrix structure and function are mostly desired; natural and synthetic polymers that can do so have been either extracted or fabricated and have been used in tissue engineering applications.
Biocompatibility, biological activity, biodegradability, and proper mechanical features of materials for specific applications determine their value in tissue engineering field. In our laboratory we aim to extract and/or synthesize biomaterials that can readily mimic physiological state of extra cellular matrix and optimize them for specific applications either via post-processing after extraction/production or modulation in extraction/production parameters. Then carry on the specific application as verification.